What is Mineral Carbonation?
Introduction
Mineral carbonation, also known as carbon mineralization, is a reaction where certain elements from rocks naturally react with carbon dioxide to form carbonate minerals – removing CO2 from the atmosphere.
In nature, rocks are transformed by the natural process of weathering. Geologic structures like the Grand Canyon are formed through millions of years of weathering. Weathering occurs in three different ways: physical, chemical, and biological.
When certain rocks are chemically weathered, through natural exposure to air and rainwater, they dissolve, releasing a portion of their elements into rainwater, some of which then bind with CO2 to form new minerals, as part of the global silicate-weathering process. The elements that are capable of binding with CO2 are members of the divalent metals group and the best suited of these divalent metals are also part of the alkaline earth group, the most abundant of which are Calcium and Magnesium. Silicate minerals, which typically contain calcium and magnesium, are common, making up 95% of the earth’s crust [1], and their natural weathering is thought to remove over 150 million tonnes of CO2 from the atmosphere each year [2]. Over millions of years, this process helps regulate the Earth’s climate by removing and storing atmospheric CO2.
In more detail, natural chemical weathering releases divalent metal ions from silicate minerals, and these ions bind with CO2 to form a new, solid carbonate mineral through the mineral carbonation reaction – safely removing and permanently storing carbon dioxide from the atmosphere. Exterra’s approach – known as engineered carbonation – parallels this natural process but uses technology to minimize the time it takes for a rock to mineralize CO2. This approach is similar to enhanced rock weathering (ERW) but occurs entirely in an engineered system, allowing for greater process control and simplified monitoring, reporting, and verification (MRV). Both engineered carbonation and ERW are specific strategies within the broader category of carbon mineralization.
Accelerating mineral carbonation
Unlike natural processes, which occur over geologic timescales, technology can be used to carry out the mineral carbonation reaction in a matter of hours. For the reaction to occur, divalent metals must be freed from the chemical structure of the rock, CO2 must be available, and the reaction environment must not be acidic. Speeding up large-scale mineral carbonation requires two major components: large quantities of well-suited rocks and an activity/process that accelerates the mineral carbonation reaction.
The proportion of divalent metals contained within a rock feedstock determines the amount of carbon which can be mineralized, while the form of those divalent metals drives the efficiency of the reaction. Even though divalent metals are abundant in many rocks, the chemical structure of those rocks often requires significant effort to extract the metal from the mineral. Potential feedstocks suitable for ex-situ mineral carbonation include:
- Natural minerals – including olivine, serpentine, and wollastonite
- Tailings – from mines including chrysotile, nickel, kimberlite, platinum group metals
- Metallurgical slags – including steel and nickel refining slags
An ideal feedstock material is available in significant quantities and contains a high proportion of labile divalent metals. Accelerating mineral carbonation requires exposing rocks to CO2 and managing the conditions of the reaction environment. CO2 exposure can come directly from the air or can be concentrated beforehand, through existing CO2-rich gases from industrial processes. When CO2 is supplied by air alone, mineral carbonation occurs slower than when the CO2 is concentrated. In engineered systems, other factors like particle size, temperature, pressure, water, and pH can also be optimized to accelerate mineral carbonation.

Ex-situ Engineered Carbonation
Ex-situ means “away from its natural condition”, referring to mineral carbonation that takes place in rocks that have been extracted from their natural location and are now on surface (e.g., rocks extracted due to mining). Managing the reaction conditions, CO2 exposure, and removal performance for materials already on Earth’s surface is simpler than in-situ mineral carbonation, which looks to perform mineral carbonation using rocks buried below the earth’s surface. Ex-situ processes are simpler because the end-to-end process can be controlled, monitored, and verified.

Global mining operations have extracted and processed about 10 billion tonnes of ultramafic rocks over time and continue to extract and process about 420 million tonnes annually [3]. Most of the extracted and processed material does not hold easily extractable valuable metals and is considered waste, which is contained within permanent storage facilities. Considering all forms of mineral waste, estimates of global annual CO2 storage capacity range from 1-2 GtCO2/yr [4,5].
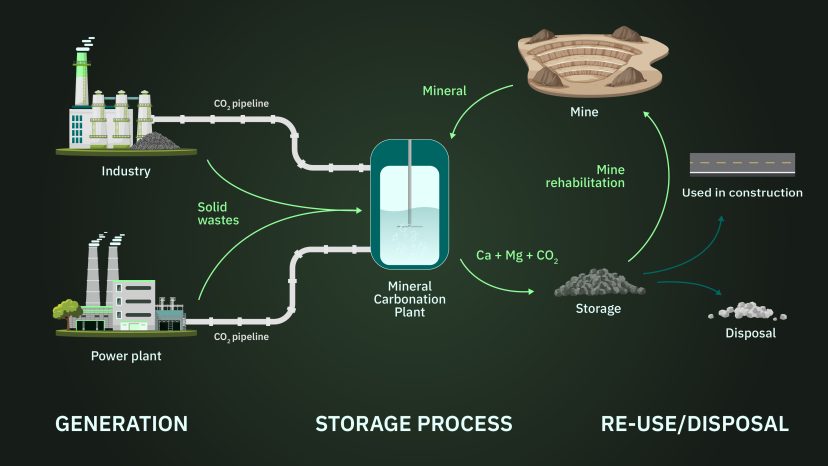
The extraction and processing of ultramafic rocks is most often linked to mining for nickel, a commodity that is expected to see increased demand as a raw material needed for the global energy transition. The generation of ultramafic mine waste from nickel and other types of mining provides ex-situ mineral carbonation projects with a material feedstock that is already crushed and ground profitably – a major cost hurdle for large-scale mineral carbonation. Minerals contained in the mine waste already undergo unintended mineral carbonation, but this reaction tends to be limited to just a fraction of its potential on relevant timescales.
Our Approach
Exterra Carbon Solutions partners with mines and mineral processors to develop projects that generate high-quality carbon credits through ex-situ engineered carbonation. Our projects implement changes to the handling and treatment of mineral waste that go beyond carbon storage to realize progress towards multiple aspects of sustainable development, including the circular economy, job creation, and ecologic benefits. Additionally, because the conditions of the reaction can be controlled, engineered carbonation projects can mineralize carbon dioxide from dilute sources including directly from flue gas.
We are currently developing carbon accounting methodologies with leading standard bodies to reliably and transparently determine the carbon benefit of accelerated ex-situ mineral carbonation projects. The methodology will enable projects to generate and sell credible carbon offsets and, ultimately, help reduce global CO2 emissions.
Sources
- Egger, A. E. (2006, April 11). The silicate minerals. Visionlearning; Visionlearning, Inc. https://www.visionlearning.com/en/library/Earth-Science/6/The-Silicate-Minerals/140
- Hilley, G. E., & Porder, S. (2008). A framework for predicting global silicate weathering and CO2 drawdown rates over geologic time-scales. Proceedings of the National Academy of Sciences of the United States of America, 105(44), 16855–16859. https://doi.org/10.1073/pnas.0801462105
- Kelemen, P. B., McQueen, N., Wilcox, J., Renforth, P., Dipple, G., & Vankeuren, A. P. (2020). Engineered carbon mineralization in ultramafic rocks for CO2 removal from air: Review and new insights. Chemical Geology, 550(119628), 119628. https://doi.org/10.1016/j.chemgeo.2020.119628
- Renforth, Phil. (2019). The negative emission potential of alkaline materials. Nature Communications, 10(1), 1401. https://doi.org/10.1038/s41467-019-09475-5
- Bullock, L. A., James, R. H., Matter, J., Renforth, P., & Teagle, D. A. H. (2021). Global carbon dioxide removal potential of waste materials from metal and Diamond mining. Frontiers in Climate, 3. https://doi.org/10.3389/fclim.2021.694175